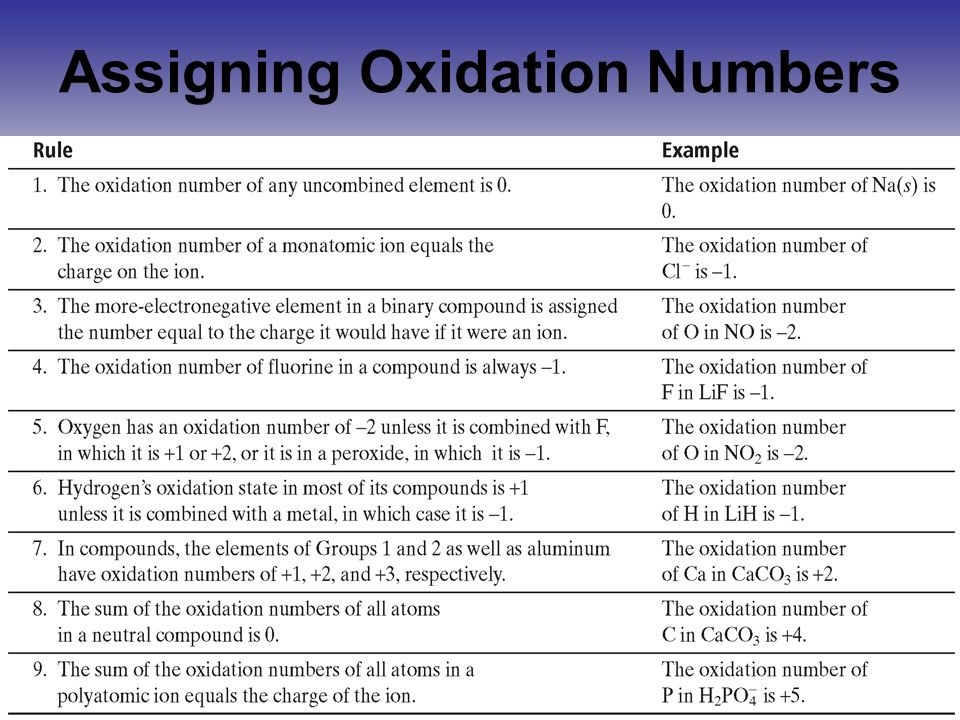
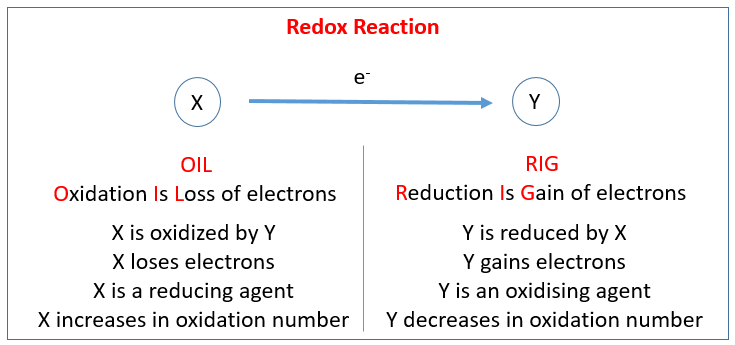
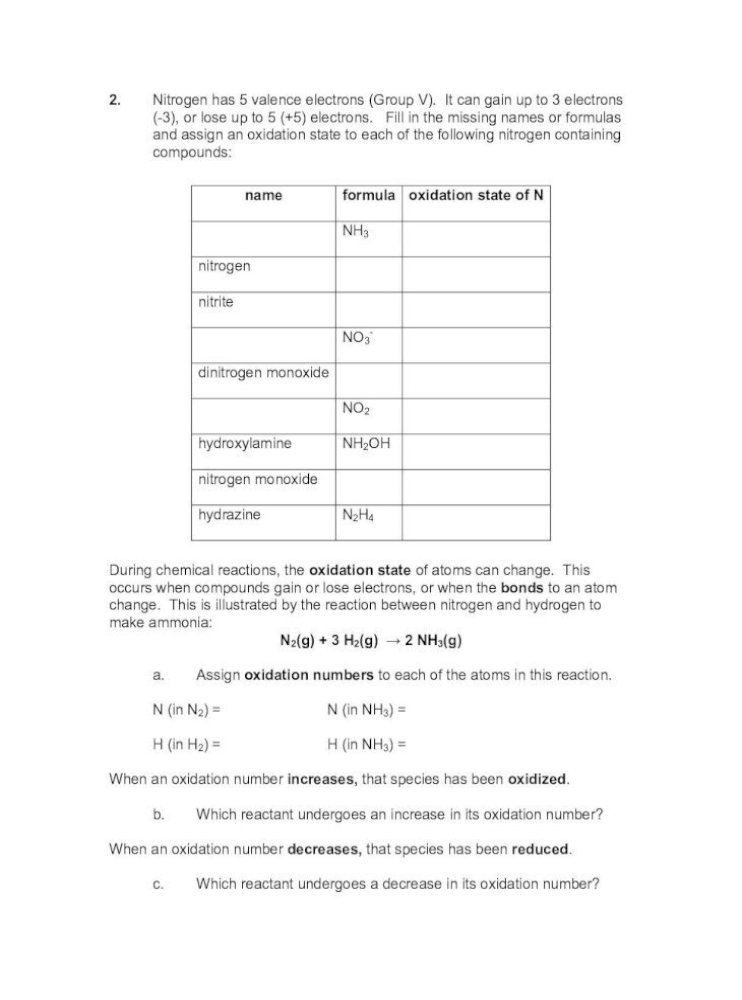
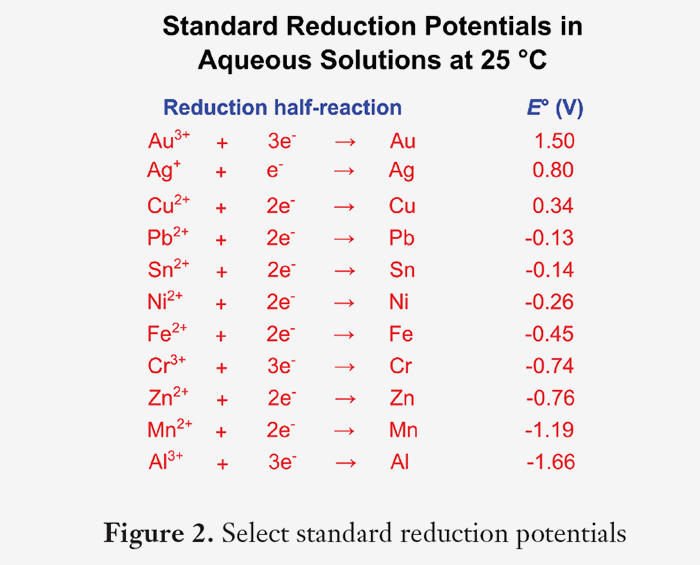
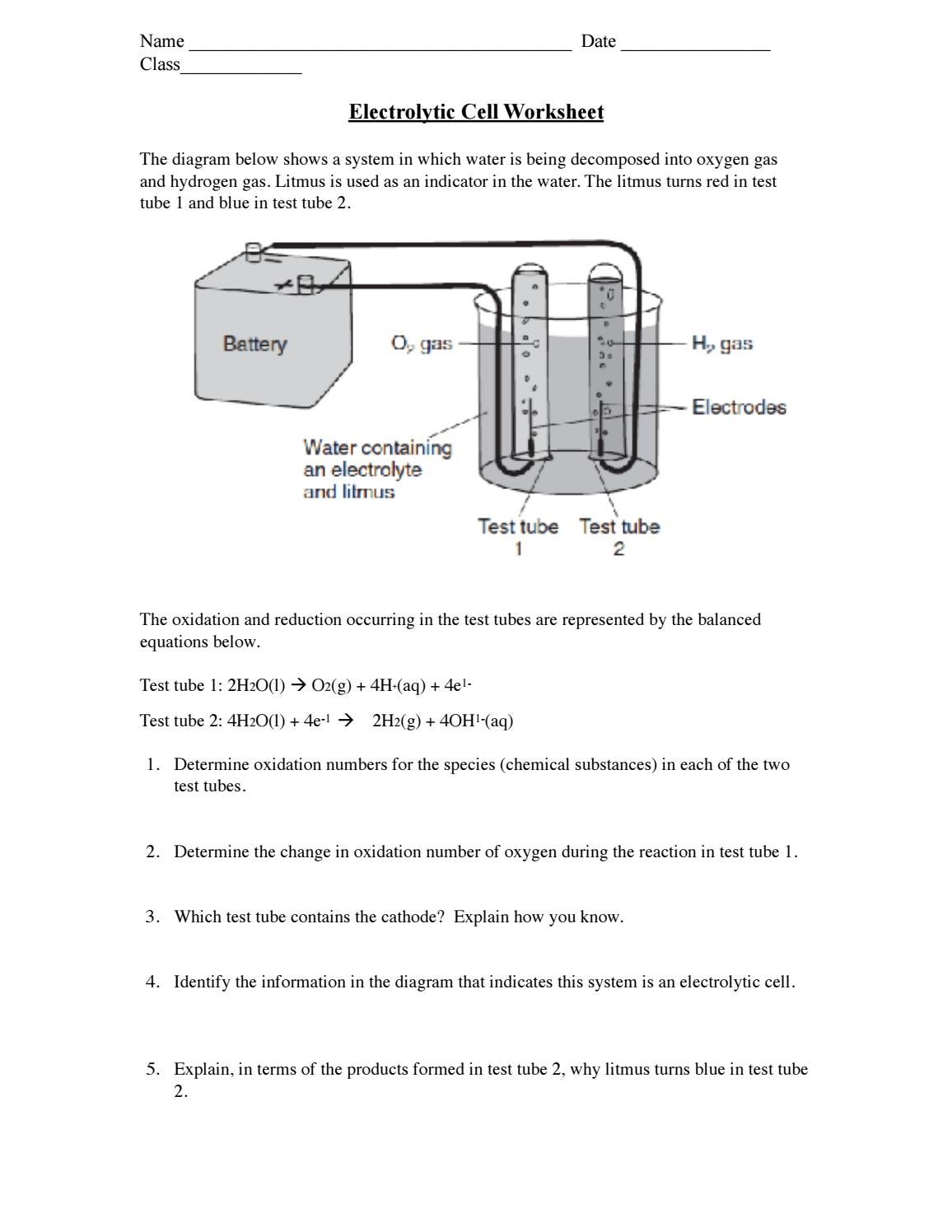
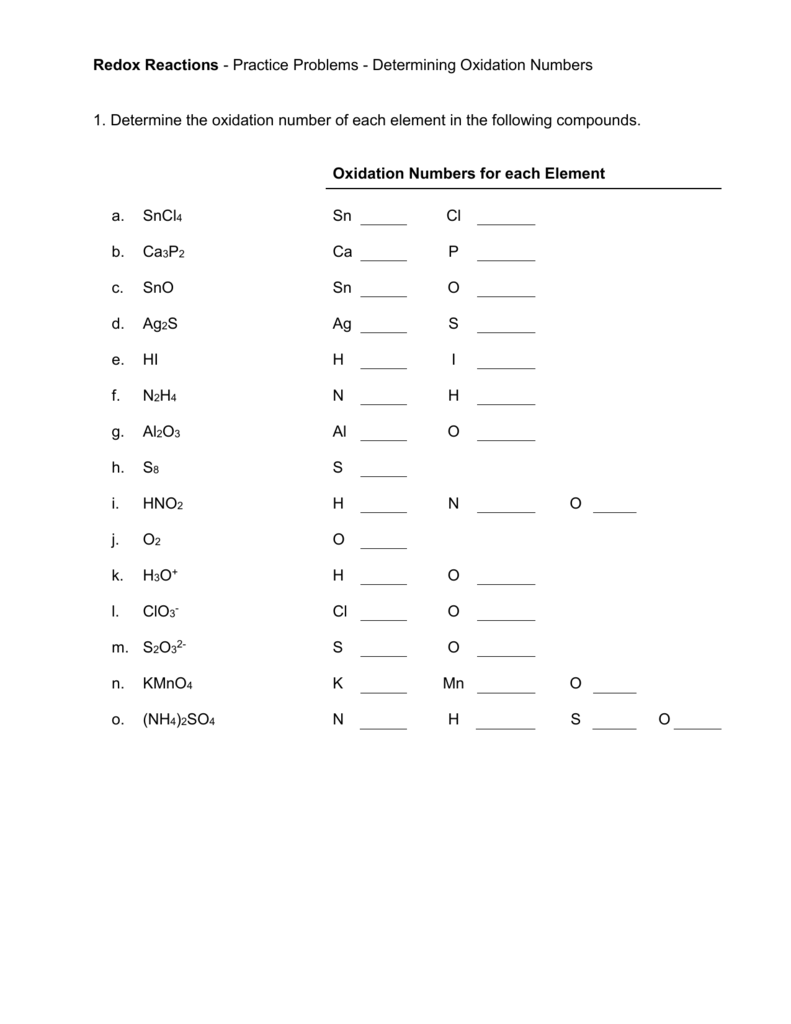
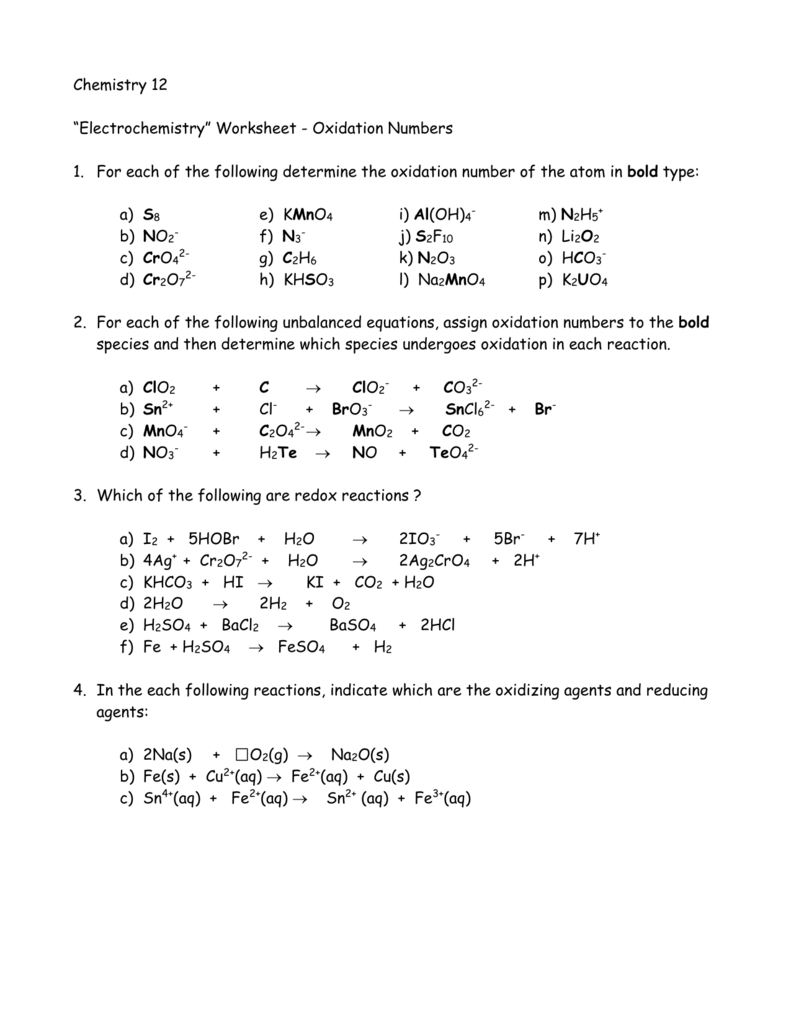
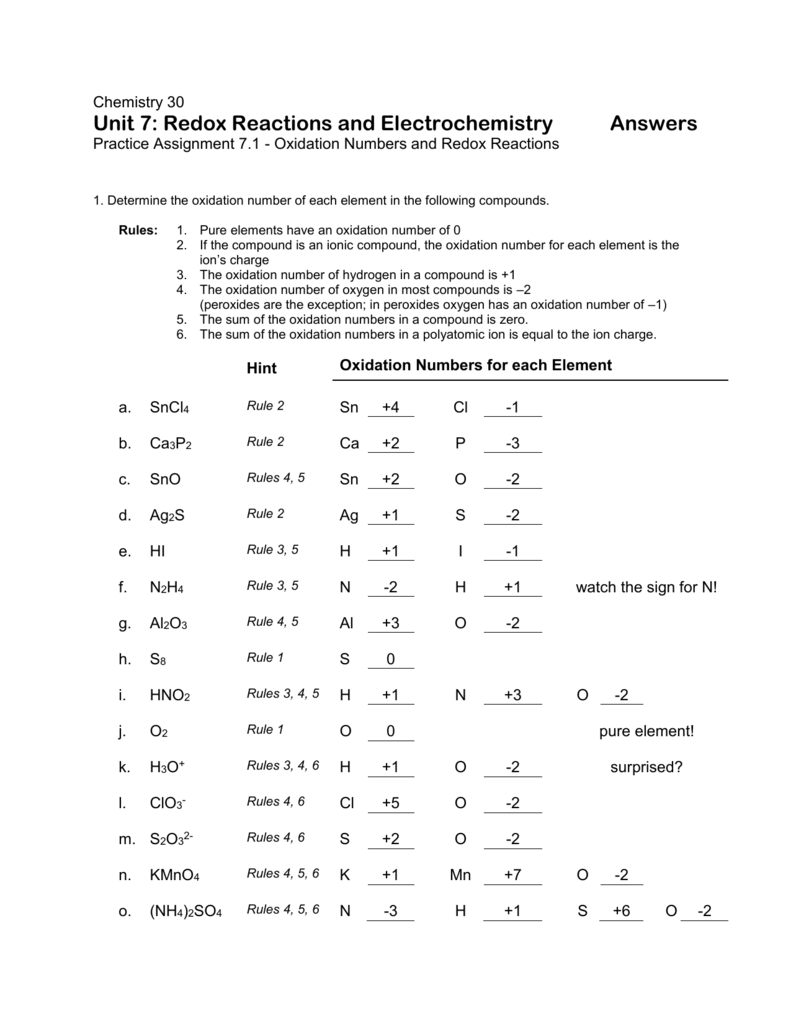
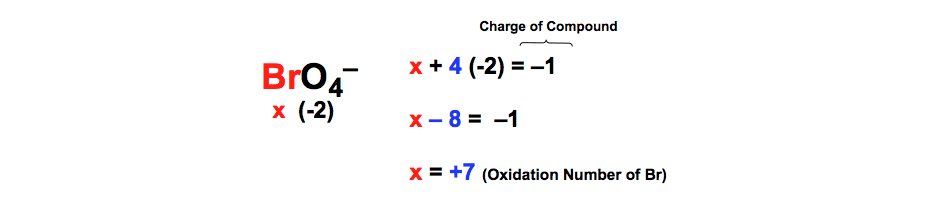Oxidation Numbers Worksheet
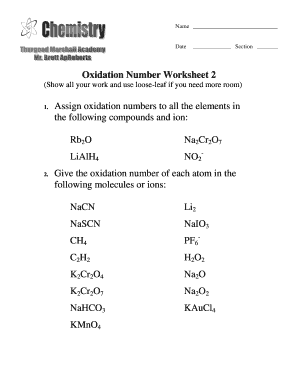
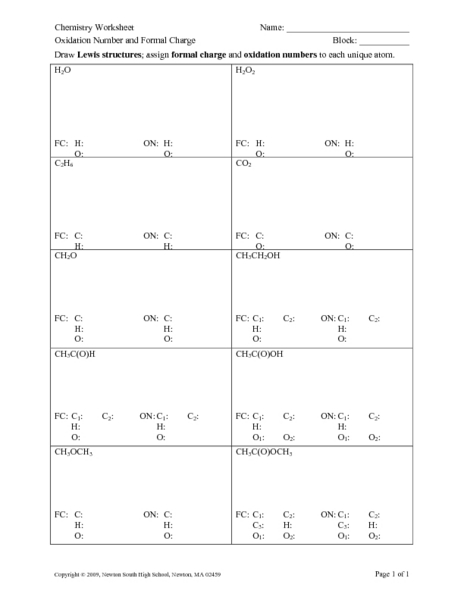
Oxidation numbers name give oxidation numbers for the underlined atoms in these molecules and ions.
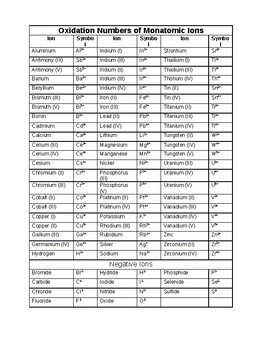
Oxidation numbers worksheet.The oxidation number of oxygen in most compounds is 2 peroxides are the exception.Redox reactions and electrochemistry.Al 2o 3 f.
Sbf 6 i.Oxidation numbers and ionic compounds write the correct formula for the compound formed by each of the following pairs of ions.Ptcl 6 2 c.
Oxidation numbers worksheet brookville k12 oh us oxidation numbers worksheet directions.Similarly the oxidation numbers for all the atoms of an ion must add up to the charge of the ion.1 z number of valence electrons on atom charge of cation 2 group number oxidation number.
4 2 6 customer reviews.The sets are mixed with easy and multi step problems.Oxidation number and redox worksheet teaching resources oxidation number and redox worksheet.
Oxidation numbers worksheets teaching resources tpt this worksheet begins with conceptual questions about oxidation numbers states.Then 6 sets of 10 gives students 60 problems to practice.For the common polyions know their charges and their names.
Oxidation numbers and ionic compounds chemistry.Pure elements have an oxidation number of 0 2.Microsoft word 14 04 oxidation numbers worksheet doc author.
H 3aso 3 h.Oct 11 2012 updated.Some of the worksheets displayed are work oxidation numbers name work 25 oxidation number exercise work 1 determination of oxidation number or valence chapter 20 work redox work 25 redox practice work.
Good mixture of problems that are direct examples of the rules and ones that are exceptions like with.Chemistry 30 unit 6.Oxidation numbers and redox worksheet.
Working out oxidation numbers and showing whether a reaction is redox or not.Brent white created date.In peroxides oxygen has an oxidation number of 1 5.
Formula element and oxidation number formula element and oxidation number 1.Ptcl 4 2 n.In a cation the oxidation number is equal to the number of these electrons which have been removed.
If the compound is an ionic compound the oxidation number for each element is the ion s charge 3.Oxidation number exercise multidict rule 1 the oxidation numbers for all the atoms in a neutral molecule must add up to 0.Oxidation numbers and electronic configurations worksheet.
You are expected to recognize polyions.Mno 4 m.Transition metal cations have a configuration d z where z is the number of valence electrons left over after ionization.
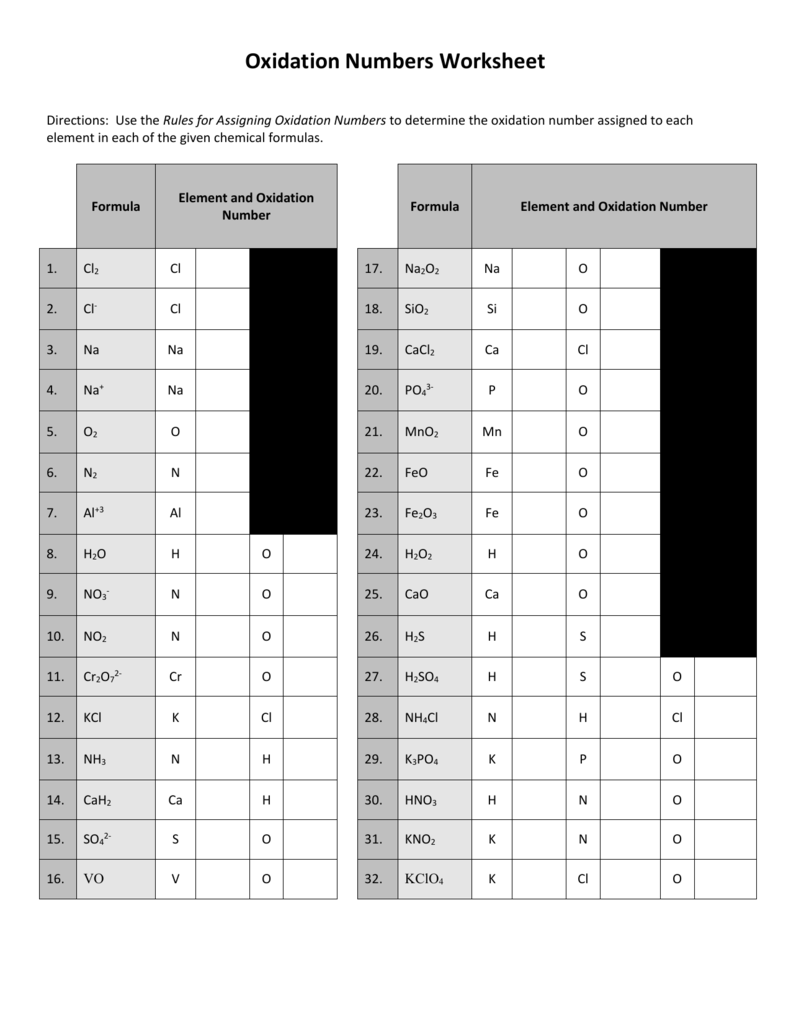
7 15 2005 10 38 28 pm.Use the rules for assigning oxidation numbersto determine the oxidation number assigned to each element in each of the given chemical formulas.





















_Oxidation_States_for_First_Row_Transition_Metals.jpg?revision=1)