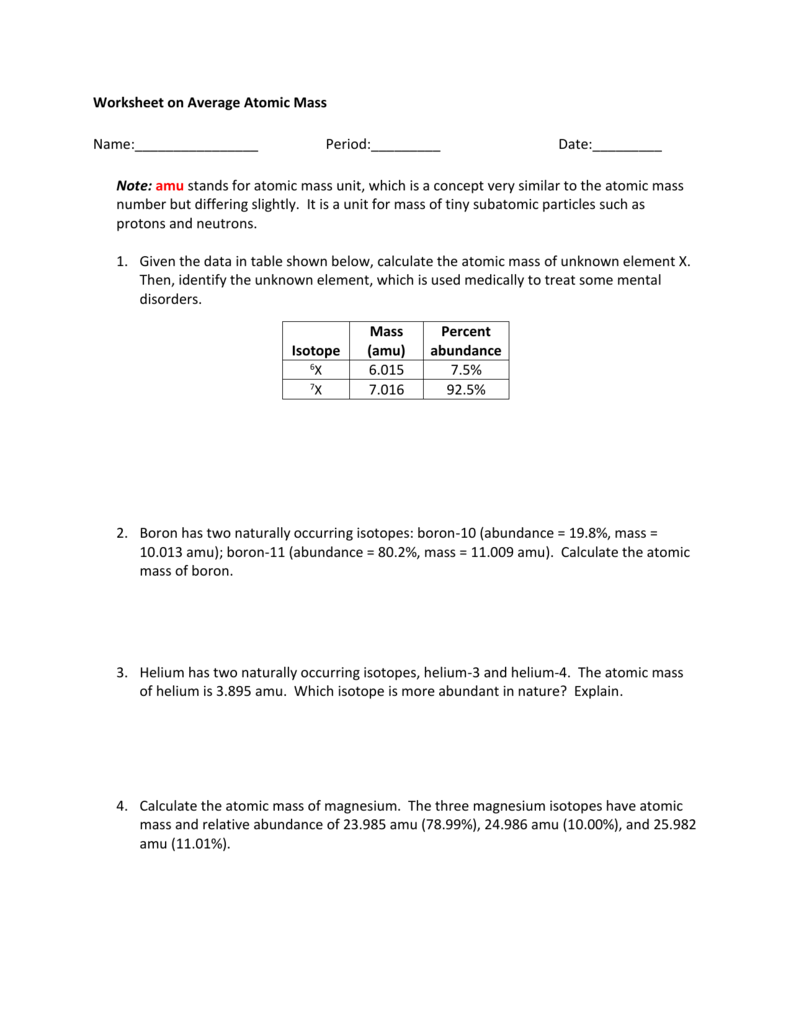
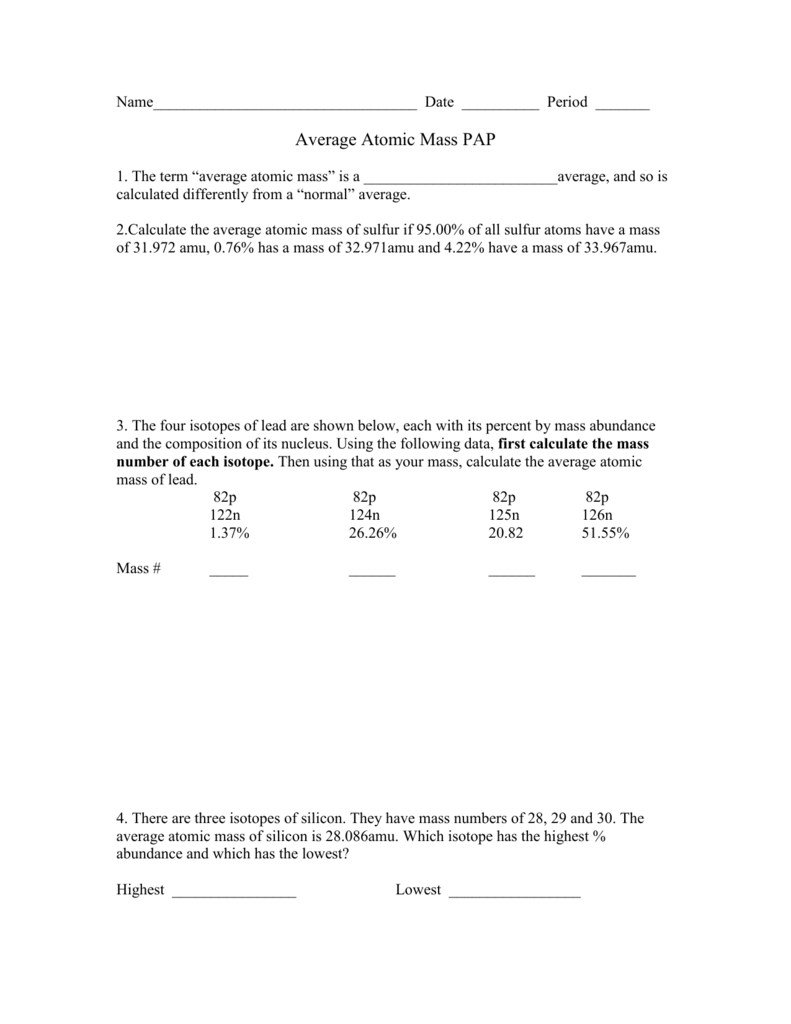
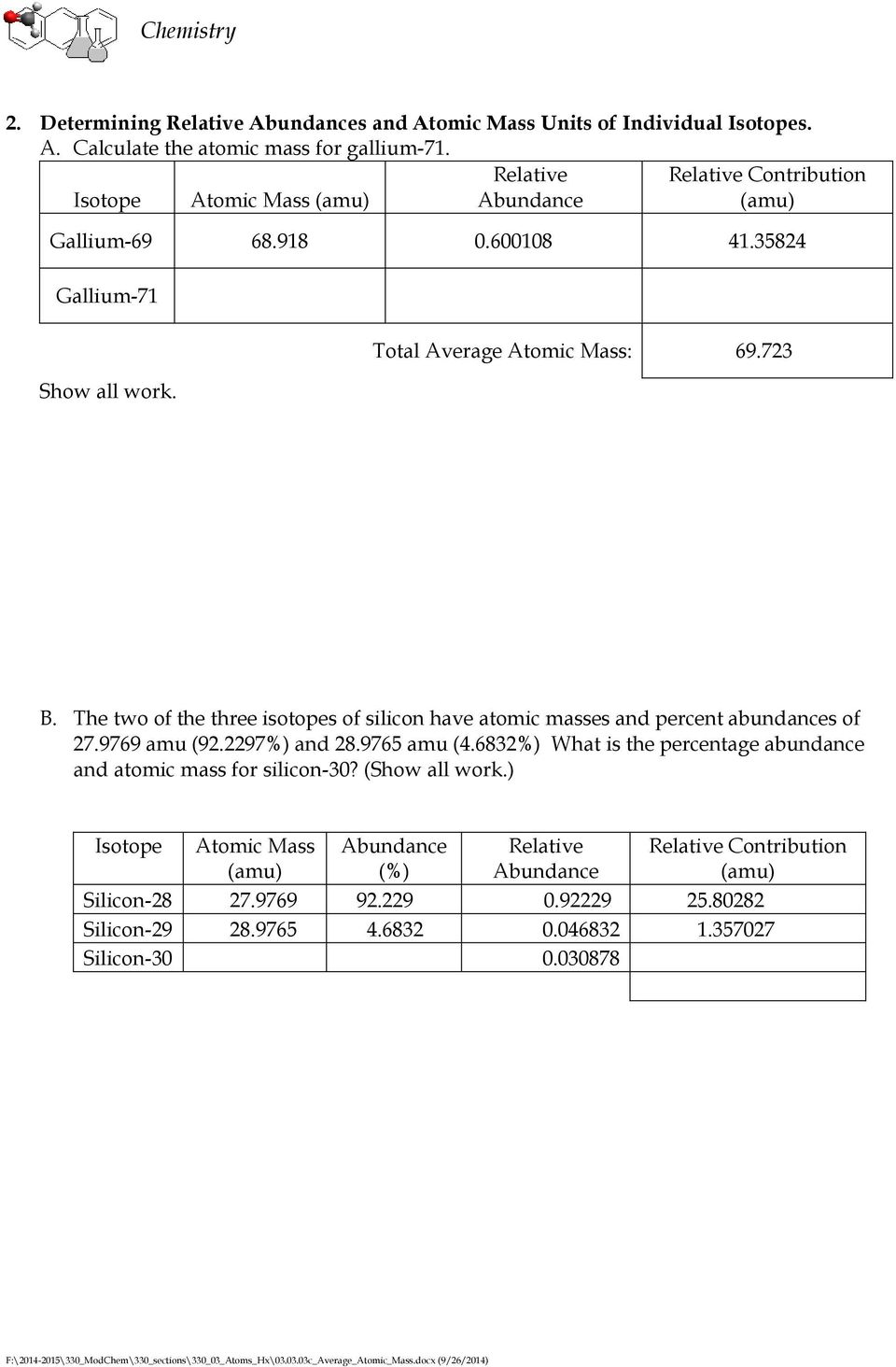
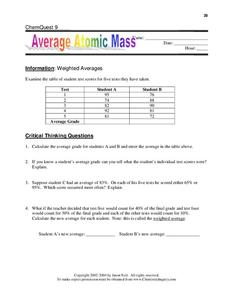
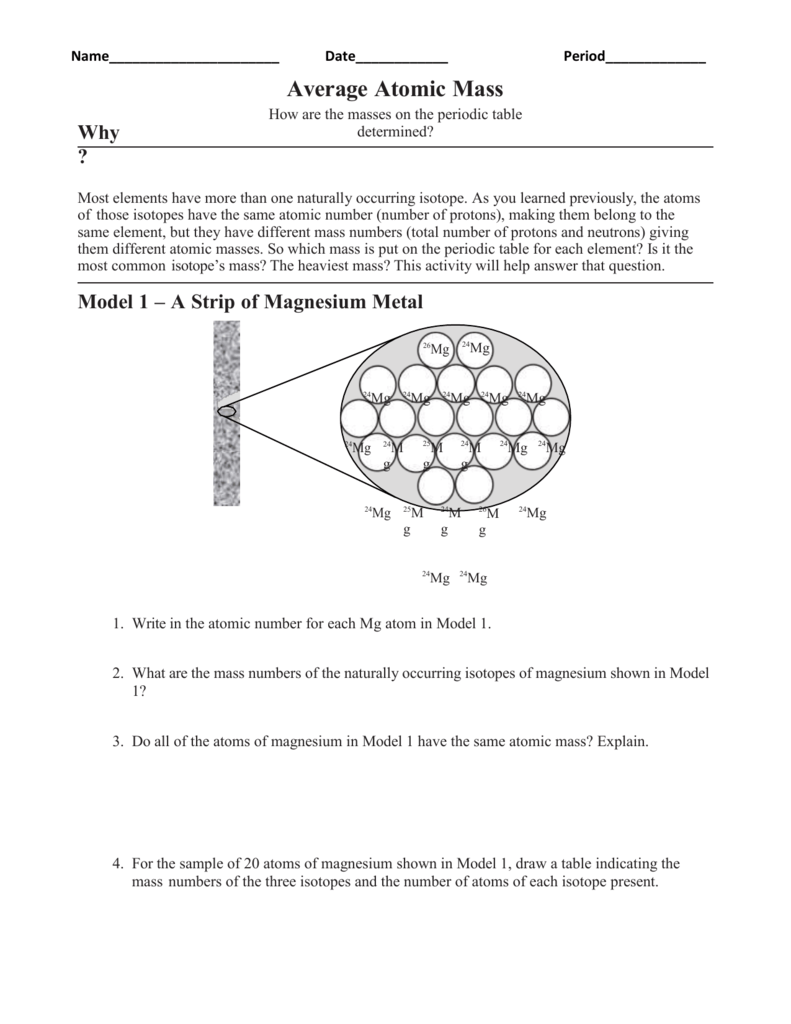
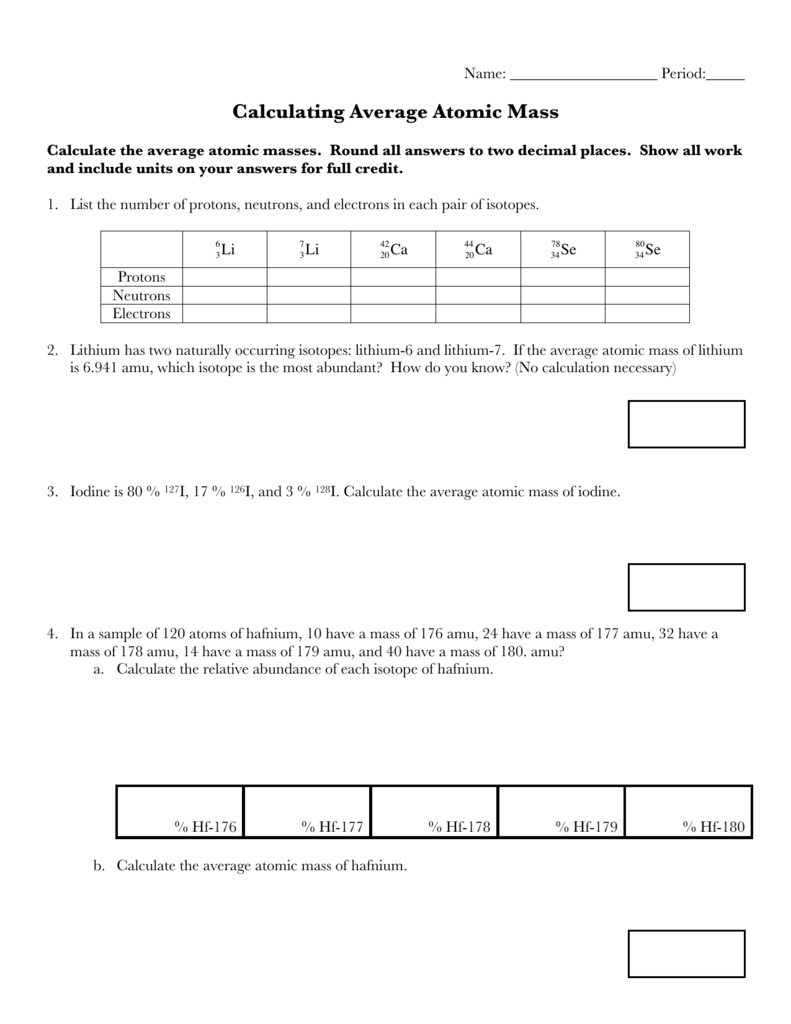
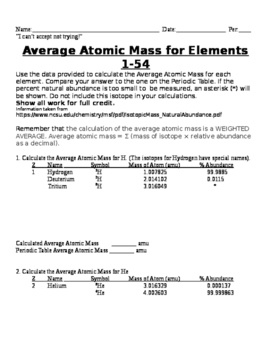
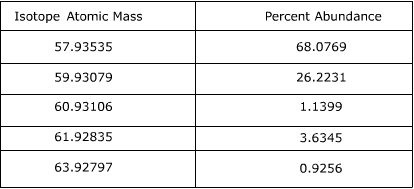Calculating Average Atomic Mass Worksheet
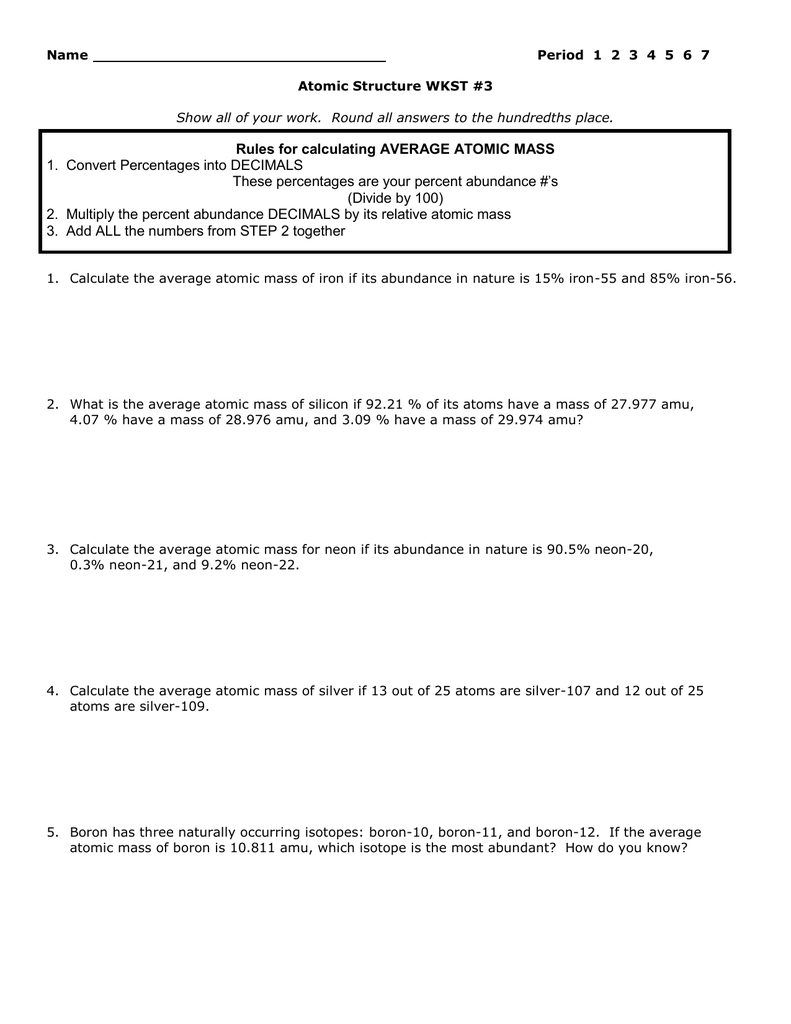
Calculate the atomic mass of carbon if the two common isotopes of carbon have masses of 12 000 amu 98 89 abundance and 13 003 amu 1 11 abundance.
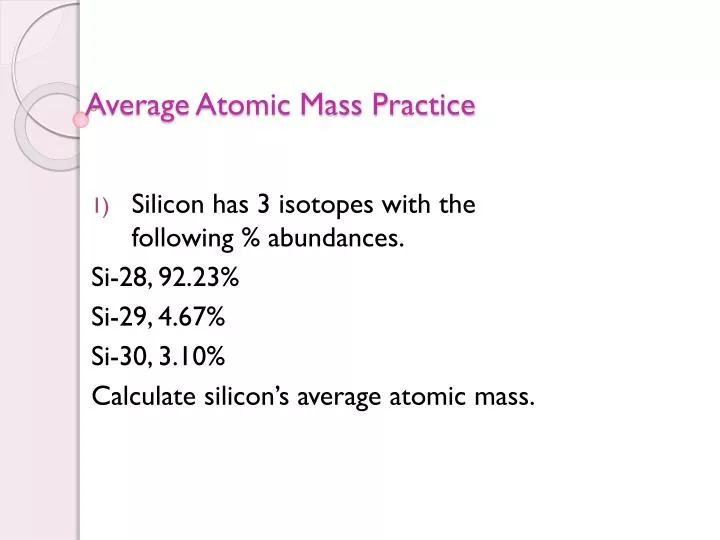
Calculating average atomic mass worksheet.A 27 97693 x 92 23 100 28 97649 x 4 68 100 29 97377 x 3 09 100 25 803 1 356 0 926 a 28 085 amu 2.Silicon 28 silicon 29 silicon 30 percent abundance.Silicon 28 92 23 27 97693 amu silicon 29 4 68 28 97649 amu silicon 30 3 09 29 97377 amu calculate the average atomic mass for the three isotopes of silicon.
The element copper has naturally occurring isotopes with mass numbers of 63 and 65.Page 3 of 4.The term average atomic mass is a average and so is calculated differently from a normal average.
The term average atomic mass is a percent average and so is calculated differently from a normal average.Name calculating average atomic mass worksheet show all calculation setups 1.Then calculate the mass numbers.
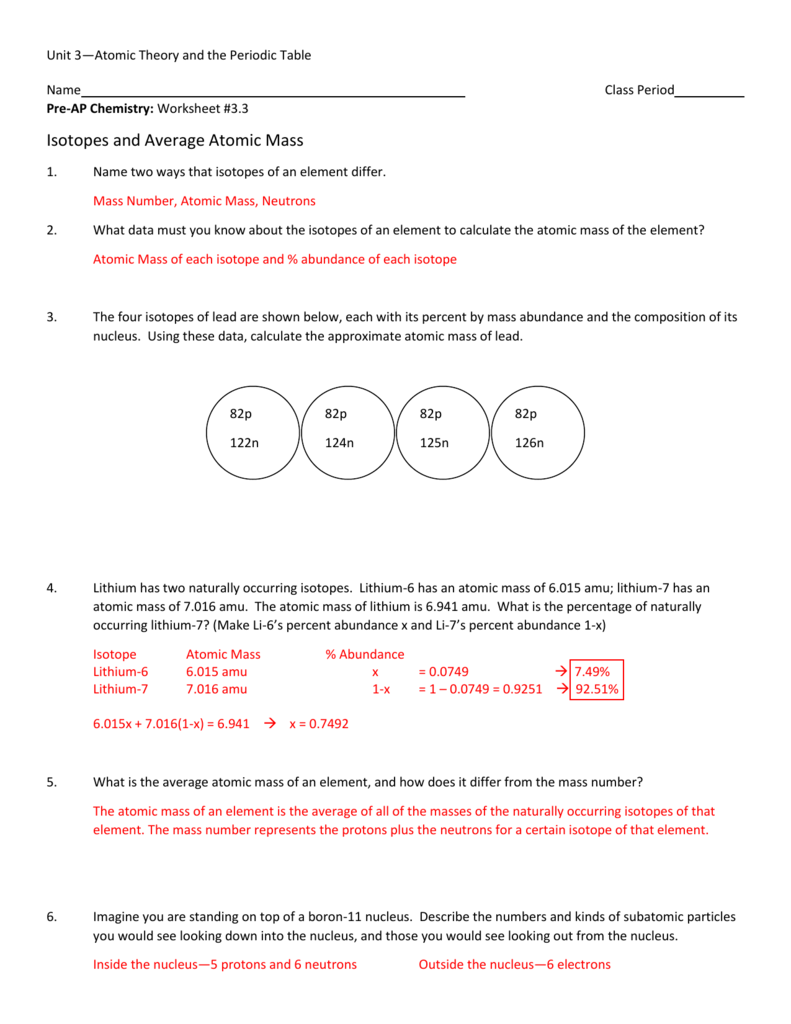
Name average atomic mass worksheet.Calculating average atomic mass worksheet worksheet the term average atomic mass is a average and so is calculated differently from a normal average.The four isotopes of lead are shown below each with its percent by mass abundance and the composition of its nucleus.
Worksheet 4 calculating atomic mass 1 doc name.Atomic mass total.Page 1 of 4.
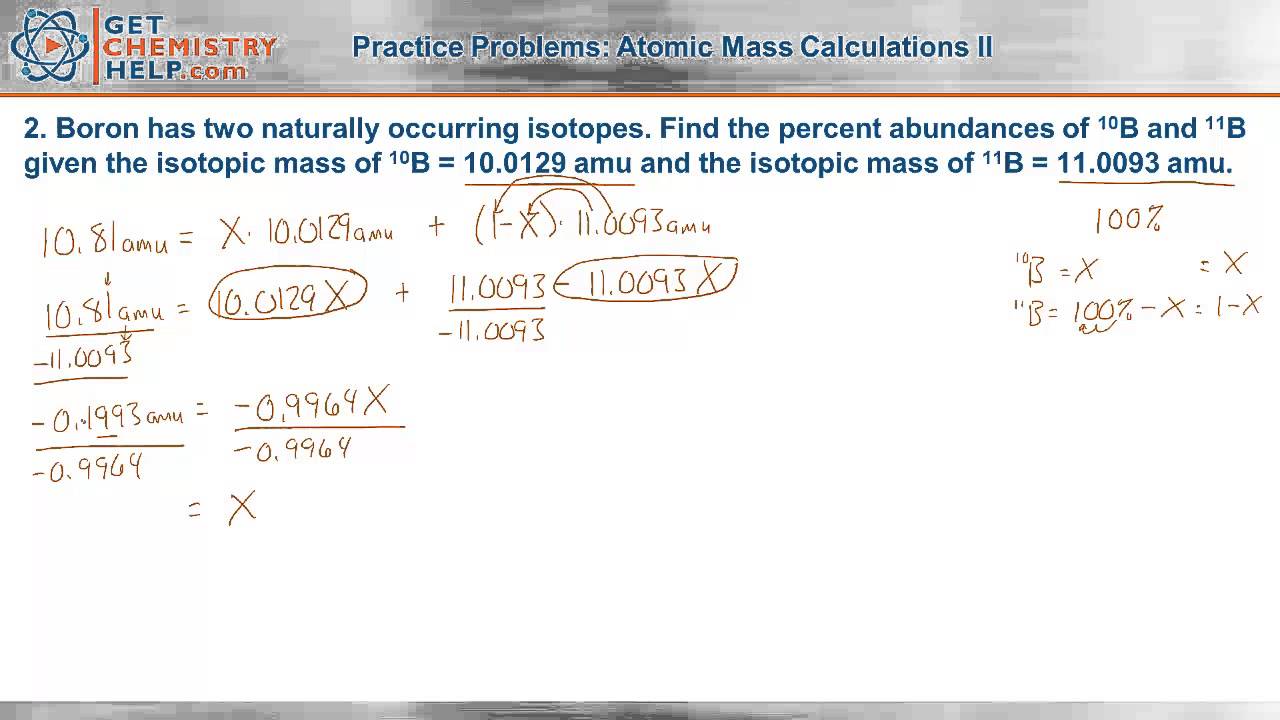
Calculating average atomic mass isotopes worksheet calculating average atomic mass isotopes worksheet calculate the average atomic mass of sulfur if 95 00 of all sulfur atoms have a mans of 31 972 amu 0 76 hos a mass of 32 97lomu and 4 22 have a mass of 33 967amu.Explain how this type of average is calculated.Pdf view id 331a9f8eb may 05 2020 by richard scarry.
Avg atomic mass doc calculating average atomic mass.1 three isotopes of silicon occur in nature.Calculating average atomic mass worksheet 1 1 docx.
Calculating average atomic mass worksheet name 1.2 two isotopes of rubidium occur naturally.Three isotopes of silicon occur in nature.
Calculate the average atomic mass of sulfur if 95 00 of all sulfur atoms have a mass of 32 0 76 has a mass of 33 and 4 22 have a mass of 34.Remember to change the abundance to a decimal.If the atomic mass of 25mg is 24 98584 amu and 26mg is 25 98259 amu calculate the actual atomic mass of 24mg.
The chlorine isotope with 18 neutrons has an abundance of 0 7577 and a mass number of 35 amu.Rubidium 85 rubidium 87 percent abundance.The four isotopes of lead are shown below each with its percent by mass abundance and the composition of its nucleus.
92 23 4 68 3 09 calculate the average atomic mass for the three isotopes of silicon.Calculating average atomic mass 2c worksheet.Formula or molar mass worksheet media publishing ebook epub kindle.
Ave atom mass abundance atomic mass abundance atomic mass mldr.Calculate the average atomic mass of copper.Page 2 of 4.
12 000 amu x 0 9889 13 003 amu x 0 0111 11 867 amu 0 144 amu 12 011 amu 2.27 97693 amu 28 97649 amu 29 97377 amu isoto es of silicon.9 y o zq q 7377 0 0.
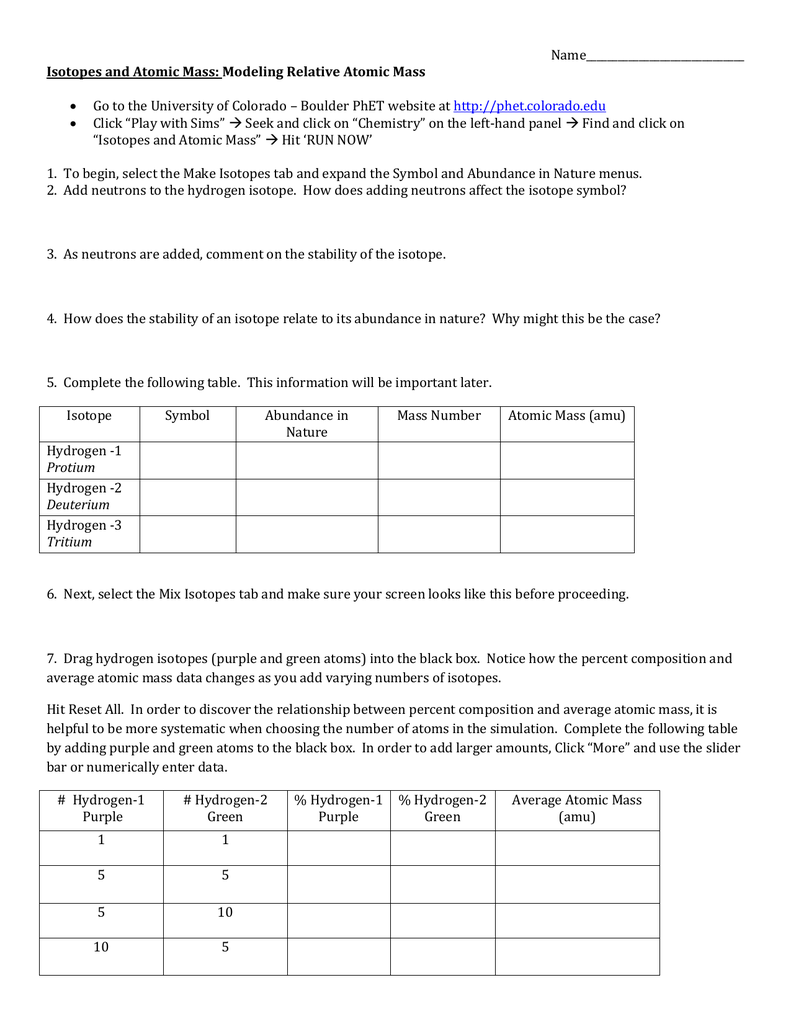
Isotopes of silicon.8 complete the table isotope mass amu relative abundance neon 20 19 992 90 51 neon 21 20 994 neon 22 9 22 avg.Average atomic mass worksheet doc name calculating.
Download the accompanying pdf worksheet calculate the average atomic mass.Using the following data first.Calculating average atomic mass worksheet nmsu calculating average atomic mass worksheet.