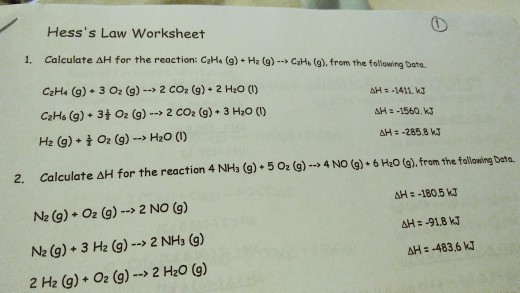Hess Law Worksheet

Hess s law worksheet chemistry libretexts hess s law worksheet last updated.
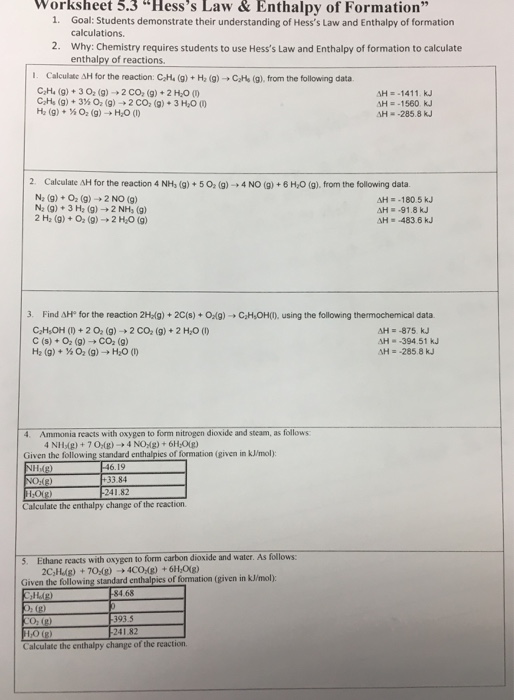
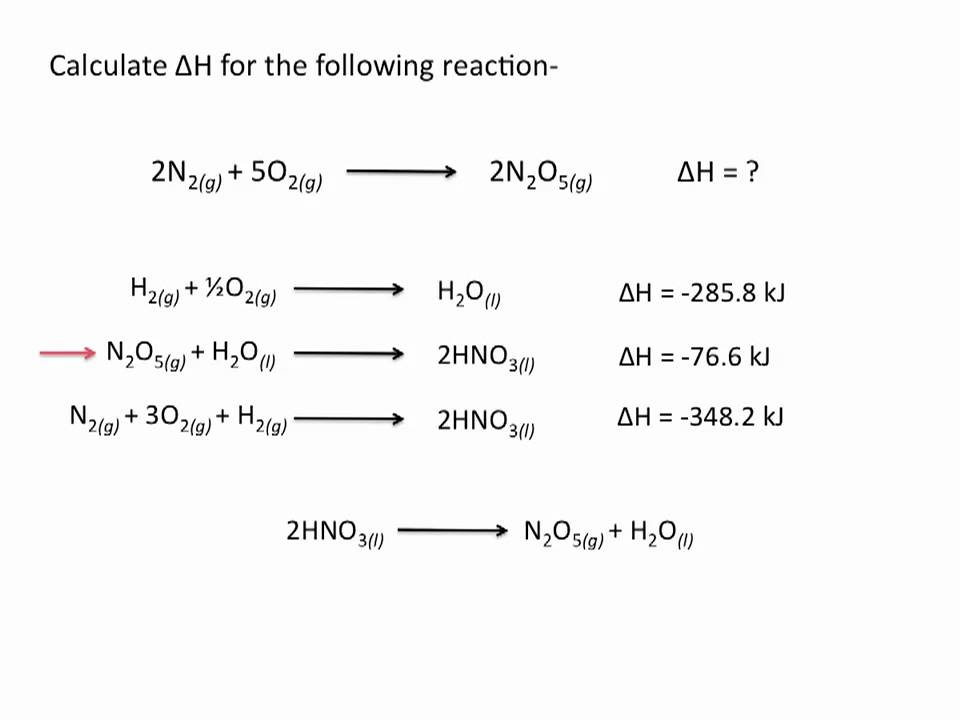
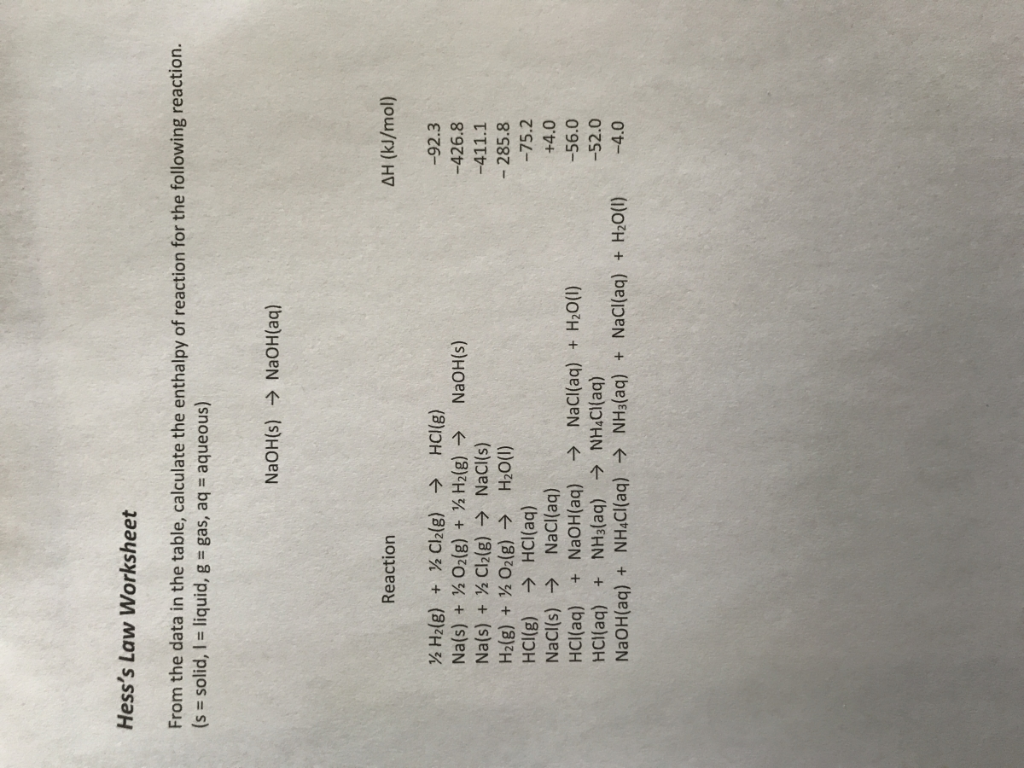
Hess law worksheet.Hess law of constant heat summation using three equations and their enthalpies germain henri hess in 1840 discovered a very useful principle which is named for him.C 2h 4 g 3 o 2 g 2 co 2 g 2 h 2o l h 1411.Hess s law hess s law for a chemical equation that can be written as the sum of two or more steps the enthalpy change for the overall equation equals the sum of the enthalpy changes of the individual steps the enthalpy change for the reaction of no2to produce n2.
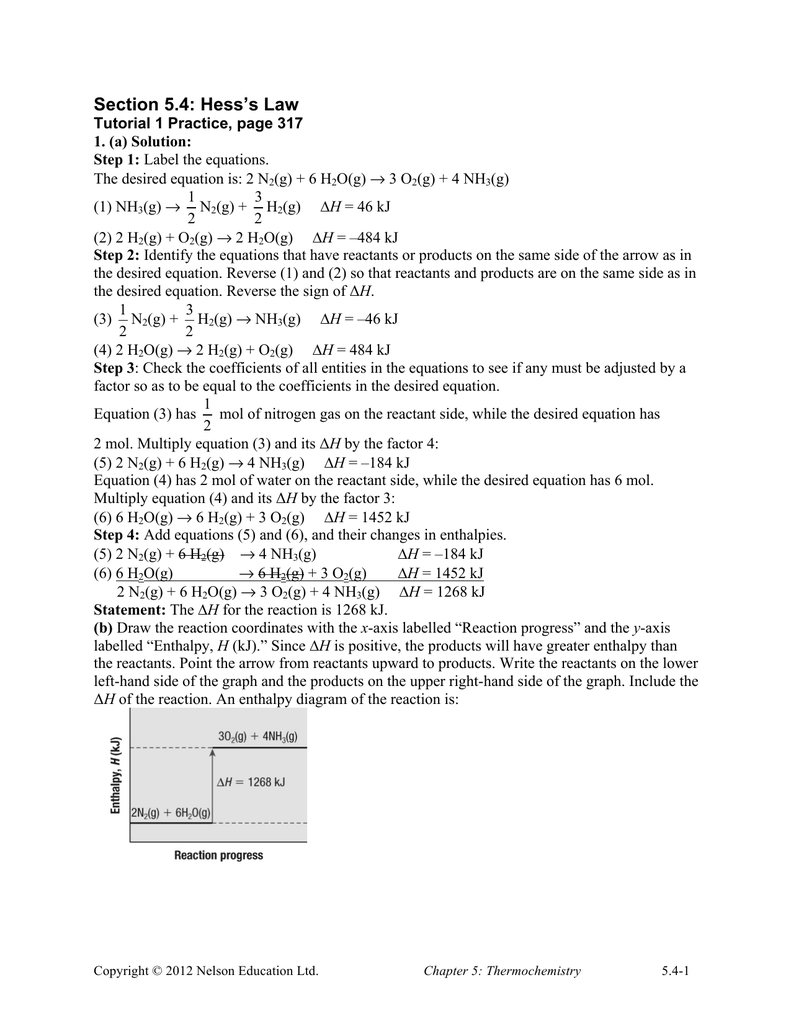
Predicting h using hess s law extra exercises student worksheet solutions predicting h using hess s law extra exercises solution lsm 5 4 3.Worksheet hess law hess law hess law states that the heat evolved in a given process can be expressed as the sum of the heats of several processes that when added yield the process of interest.Contributed by mark draganjac.
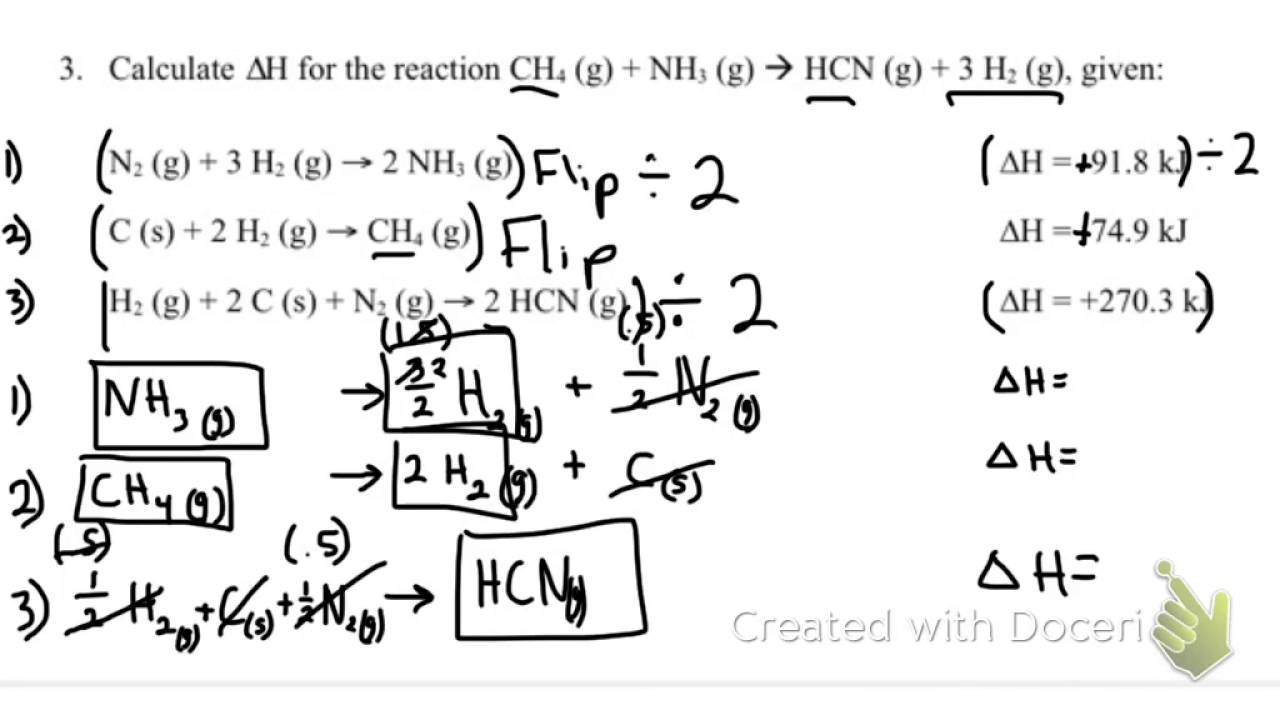
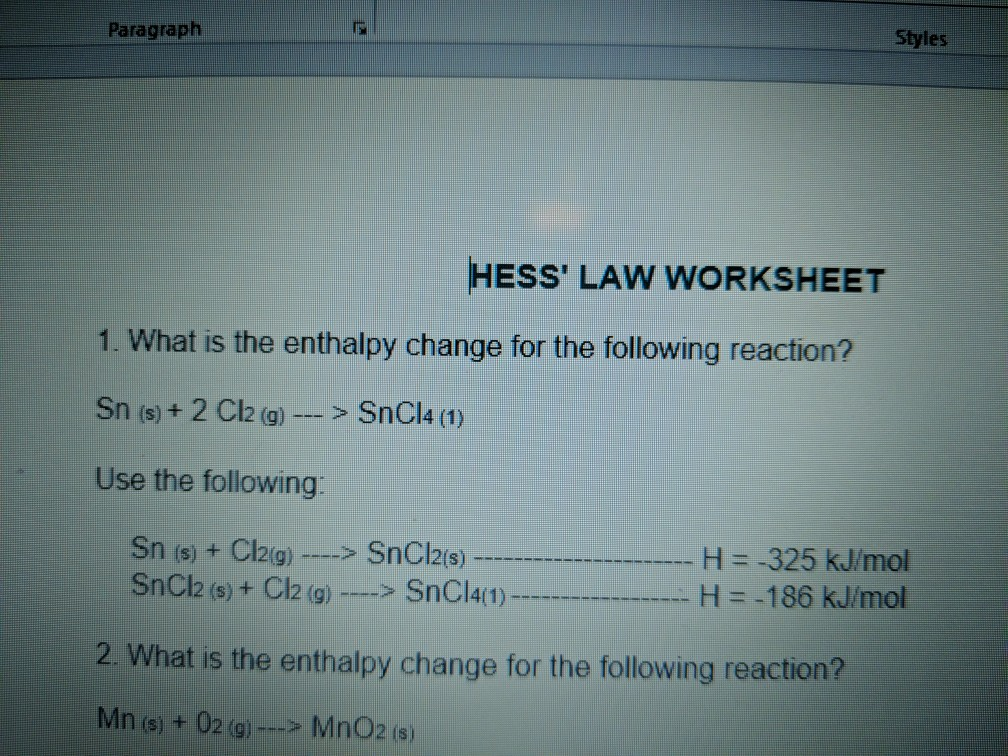
Student id work in groups on these problems.Hess s law worksheet rancho high school hess s law worksheet calculate h for the reaction.You should try to answer the questions without referring to your textbook.
Kj mole c 2h 6 g 7 2 o 2 g 2 co 2 g 3 h 2o l h 1560.Hess law worksheets kiddy math hess law displaying top 8 worksheets found for this concept.A 505 g piece of.
C2h4 g h2 g c2h6 g from the following data.10 10 2002 11 33 26 am.Professor chemistry at arkansas state university.
Kj mole h 2 g 1 2 o 2 g h 2o l h 285 8 kj mole 2.Some of the worksheets for this concept are hes law work answers work hess law hess law chemistry quiz hes law and calorimetry multiple hes law work name honors chemistry hes law example exercise henrys law chem 115 pogil work.Calculate h for the reaction c 2h 4 g h 2 g c 2h 6 g from the following data.
Hess s law worksheet answers lozon hess s law worksheet answers 1.If you get stuck try asking another group for help.If the reactants and products are the same it doesn t matter how the reaction is carried out.
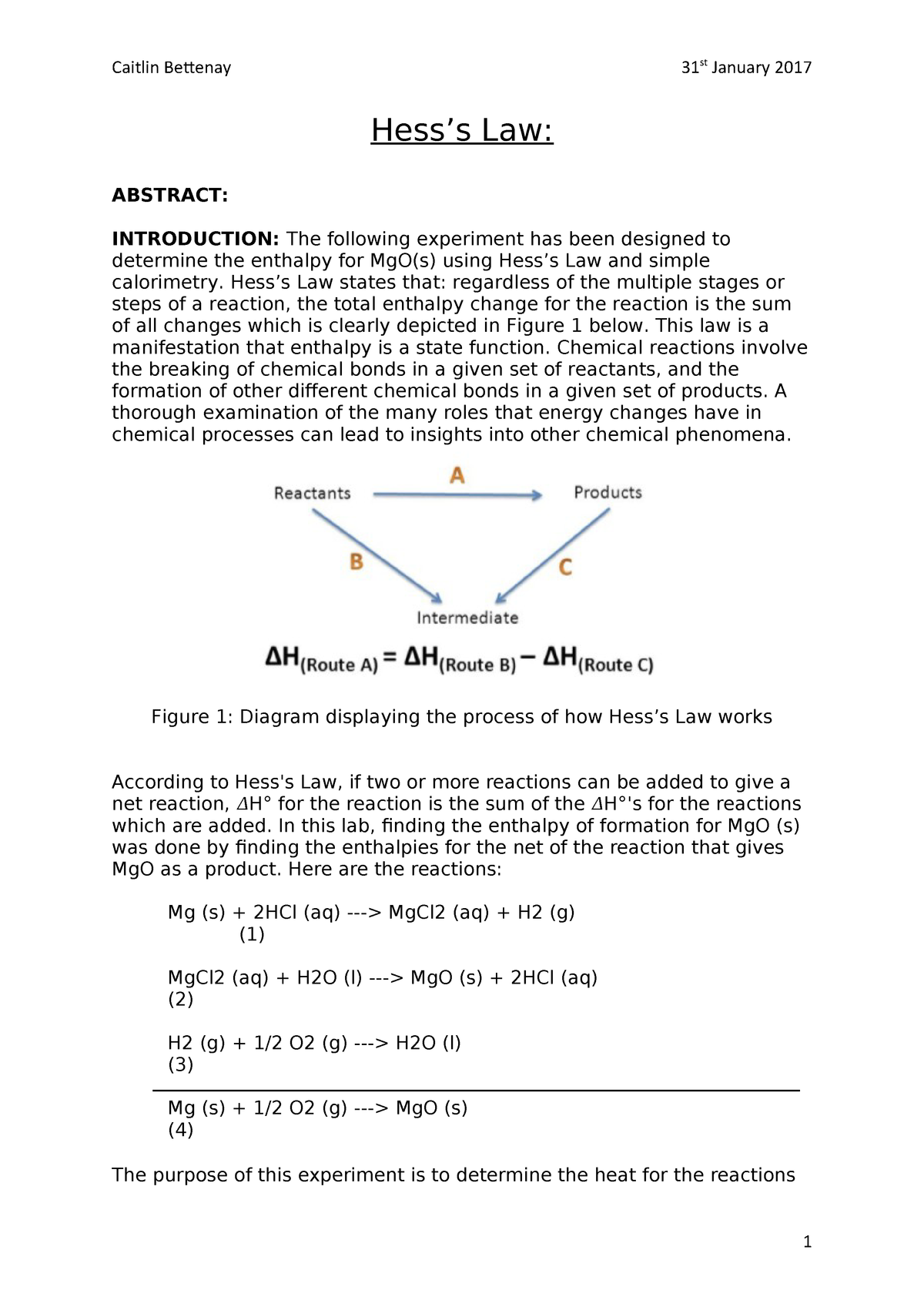
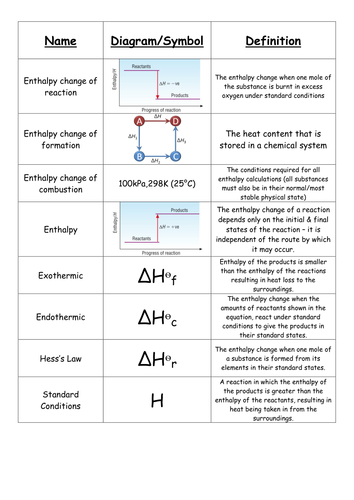
This law is a manifestation that enthalpy is a state function.Hess law using three equations and their.In other words enthalpy is a state function.
Calculate h for the reaction 4 nh 3 g 5 o 2 g 4 no g.The enthalpy of a given chemical reaction is constant regardless of the reaction happening in one step or many steps.