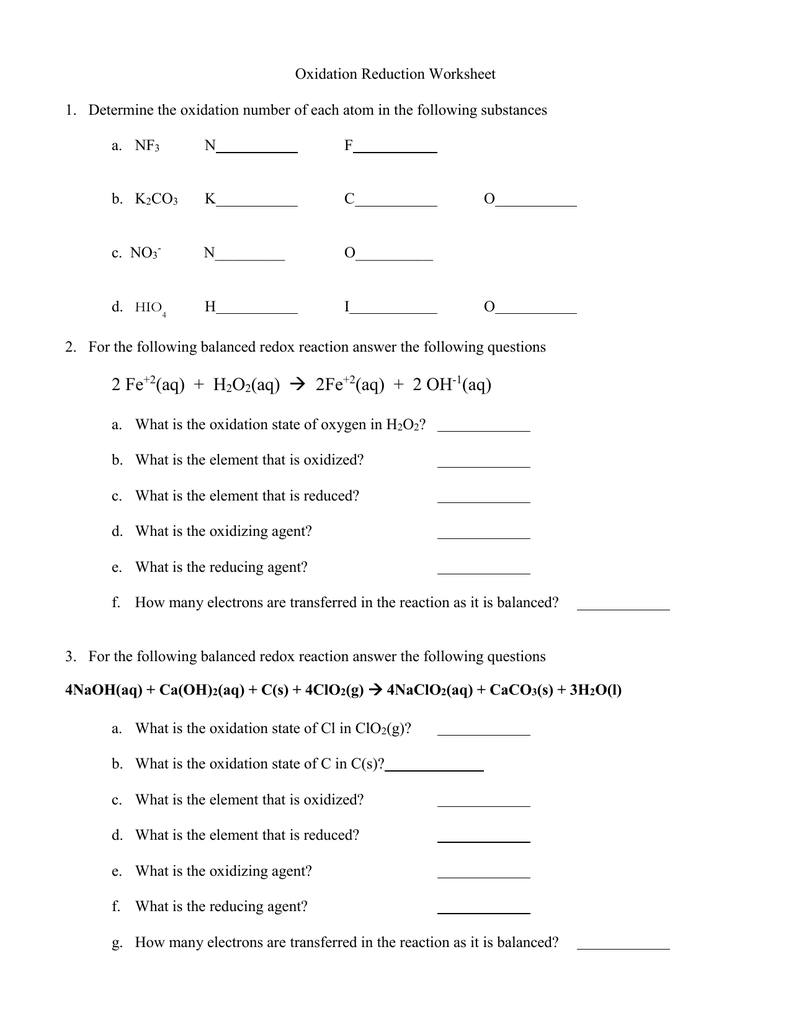
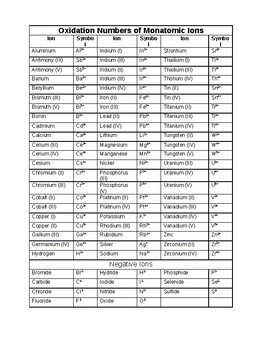
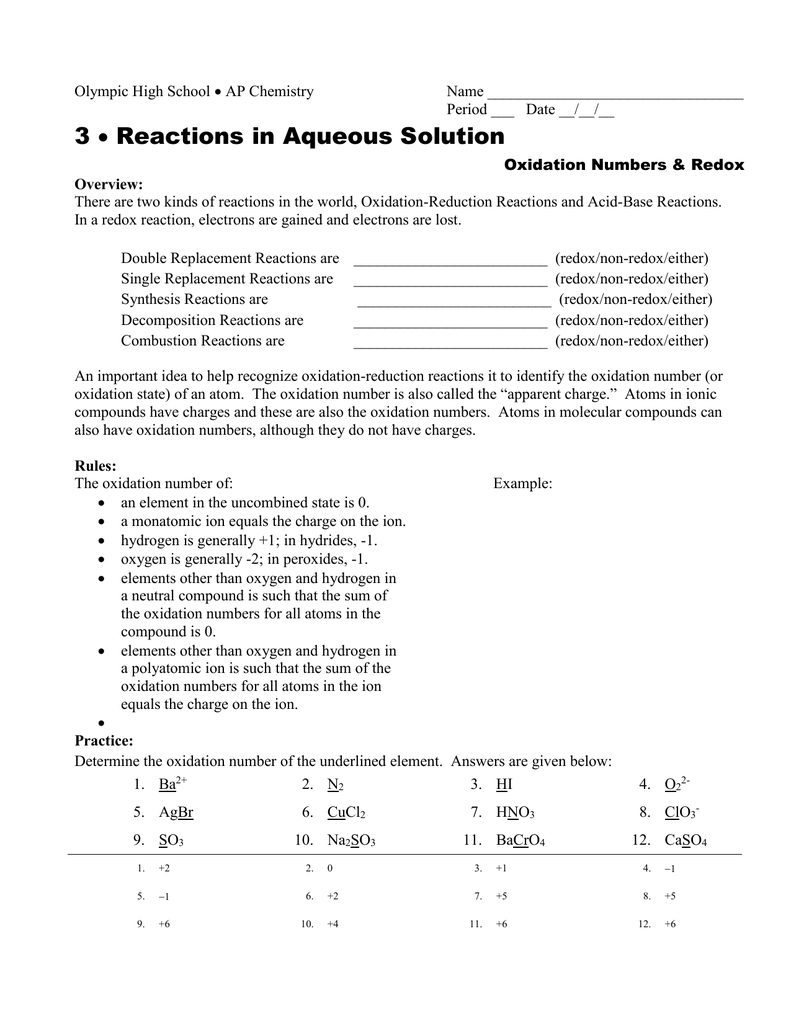
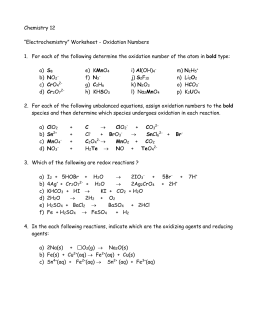
Oxidation Number Worksheet With Answers
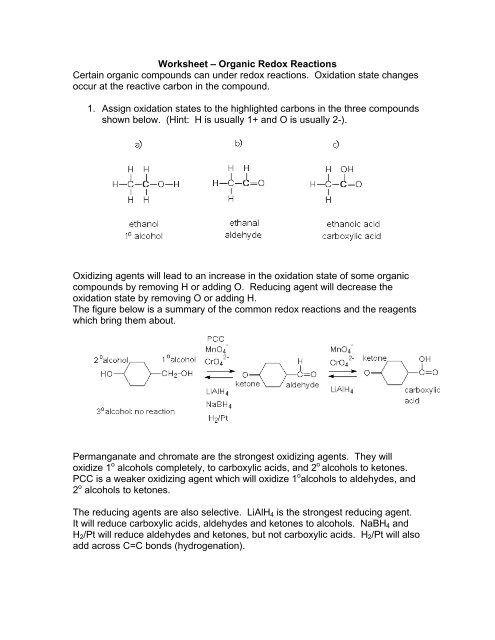
Redox reactions and electrochemistry.
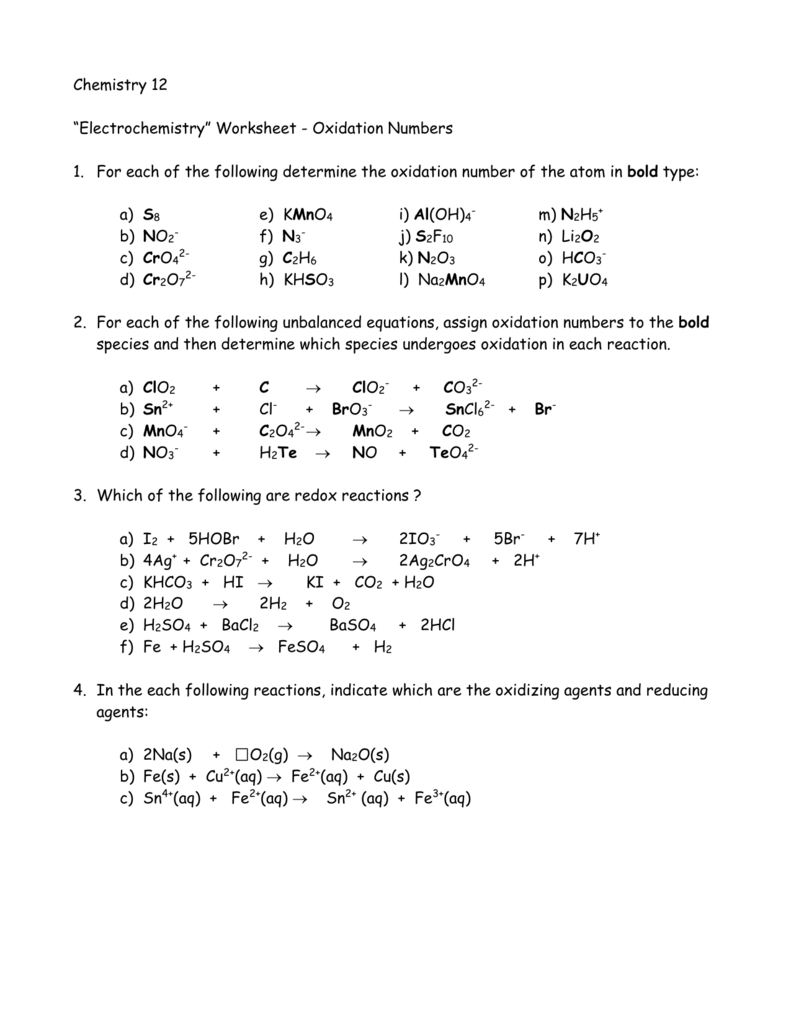
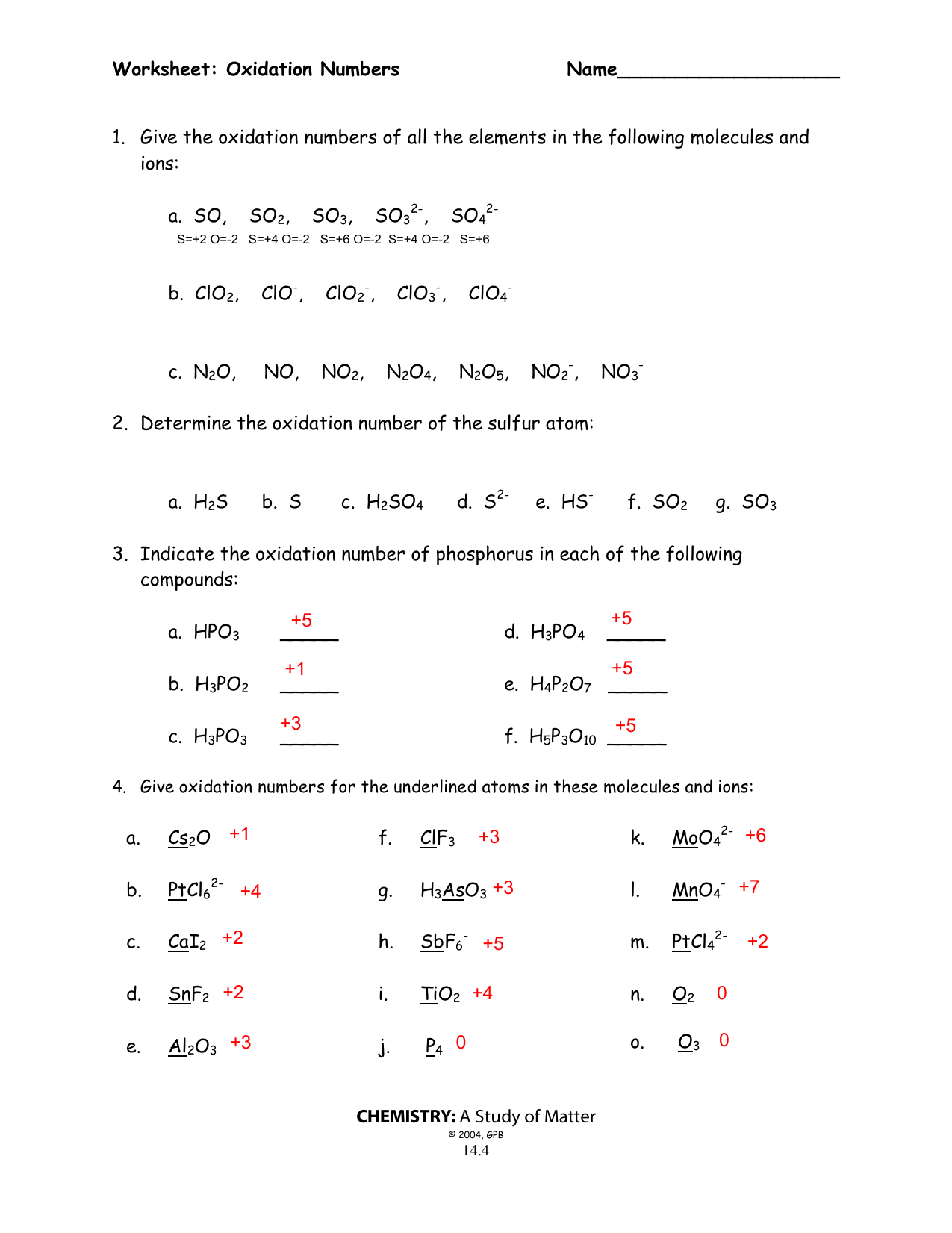
Oxidation number worksheet with answers.P 3 w 2f9 3.Oxidation numbers are either real charges or formal charges which help chemists keep track of.Microsoft word 14 04 oxidation numbers worksheet doc author.
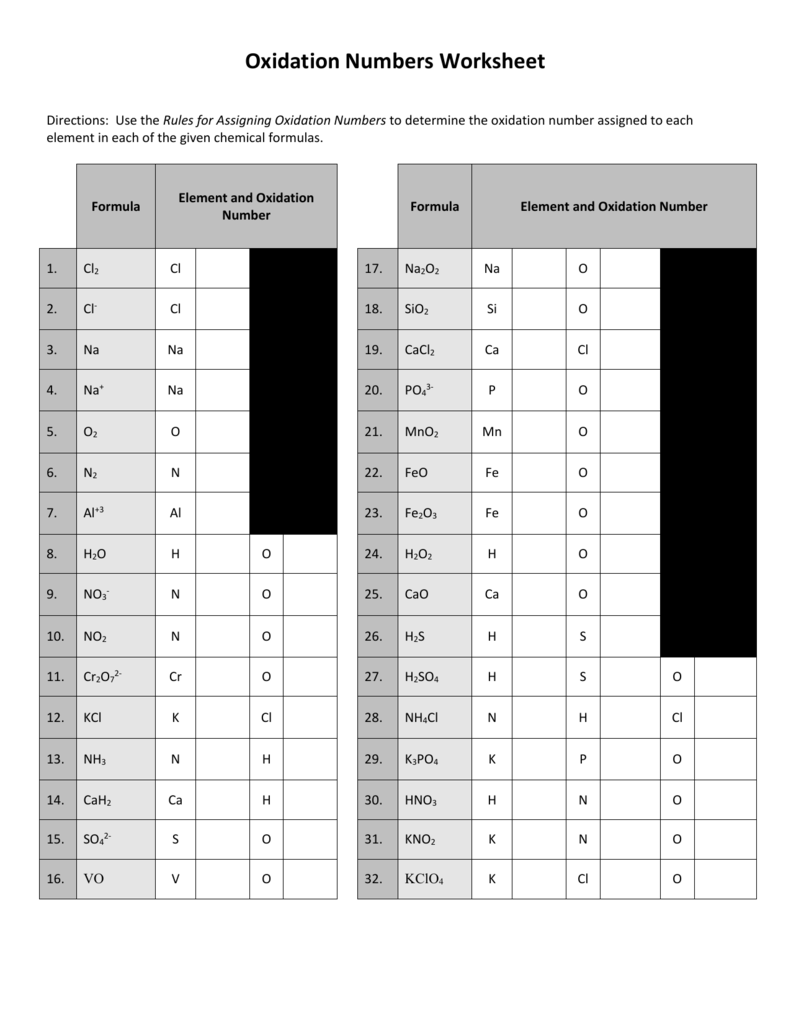
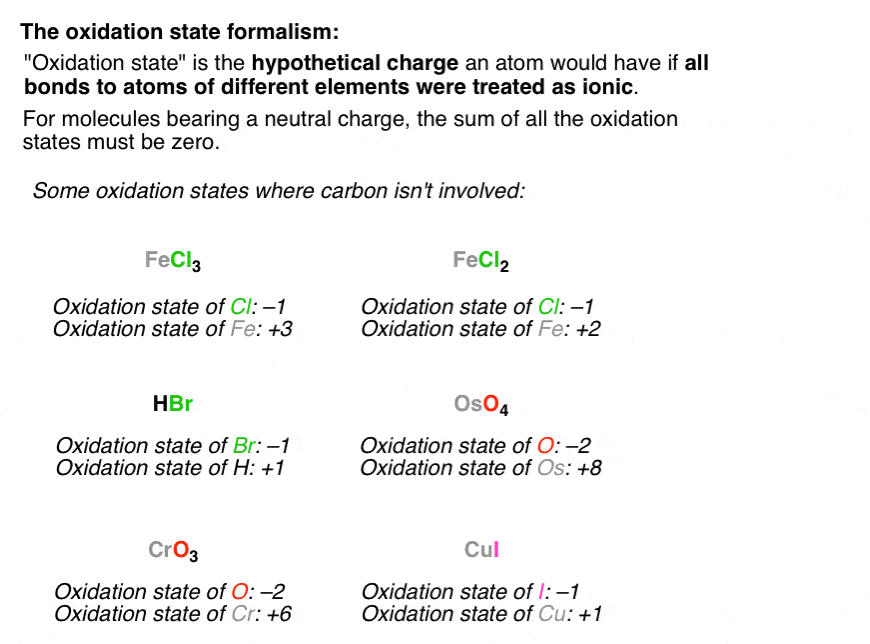
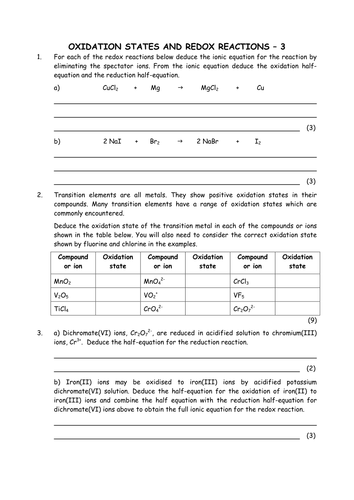
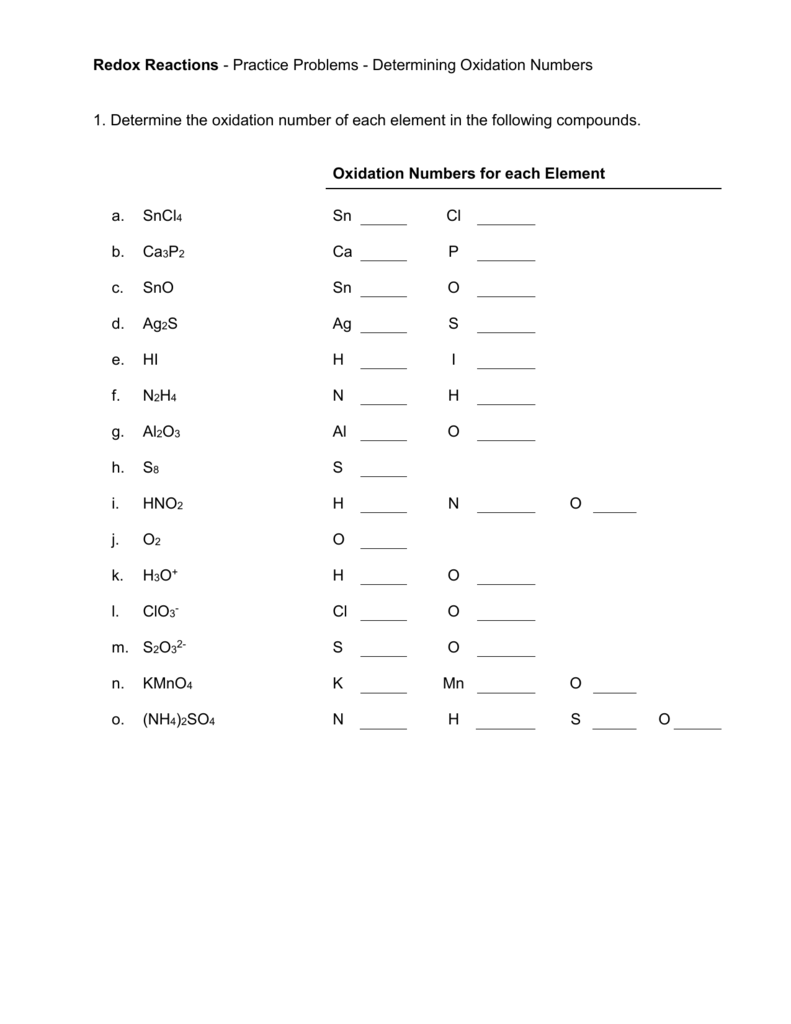
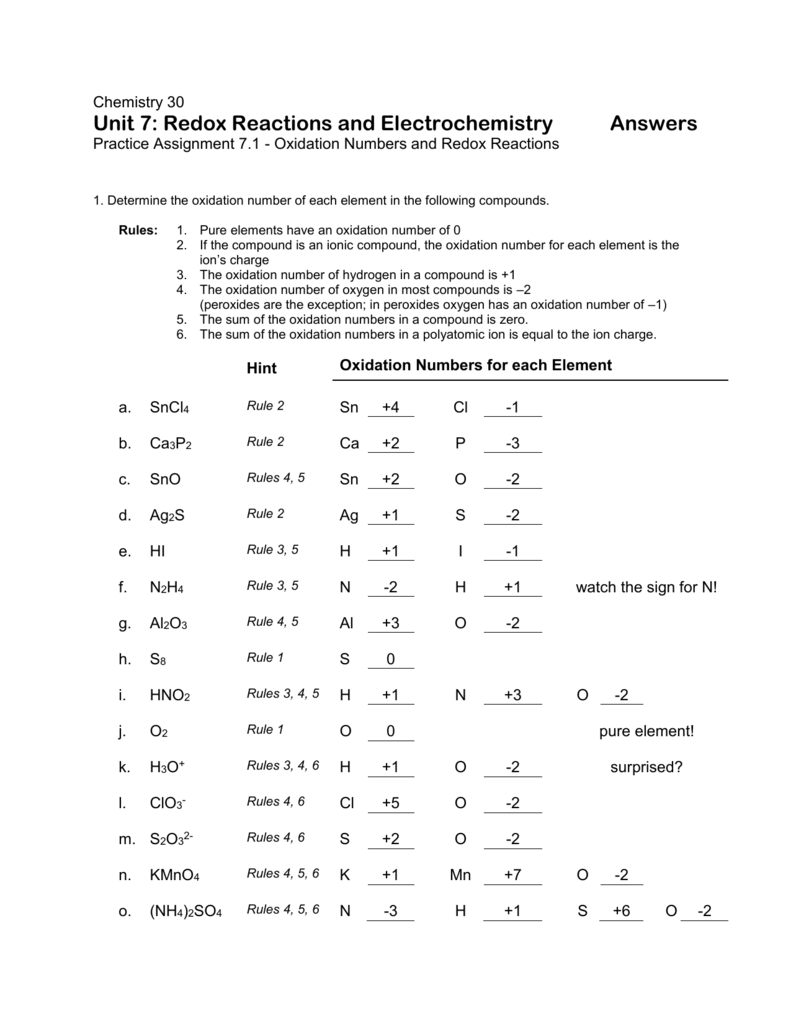
For example to find the oxidation state of sulfur in h 2 so 4 h 2 so 4 h 2 1 2 o 4 2 8 6 s.H 3aso 3 h.The oxidation number of fluorine in a compound is always 1.
Naf na 1 if 3 i 3 clf 2.Once you find your worksheet click on pop out icon or print icon to worksheet to print or download.Ptcl 6 2 c.
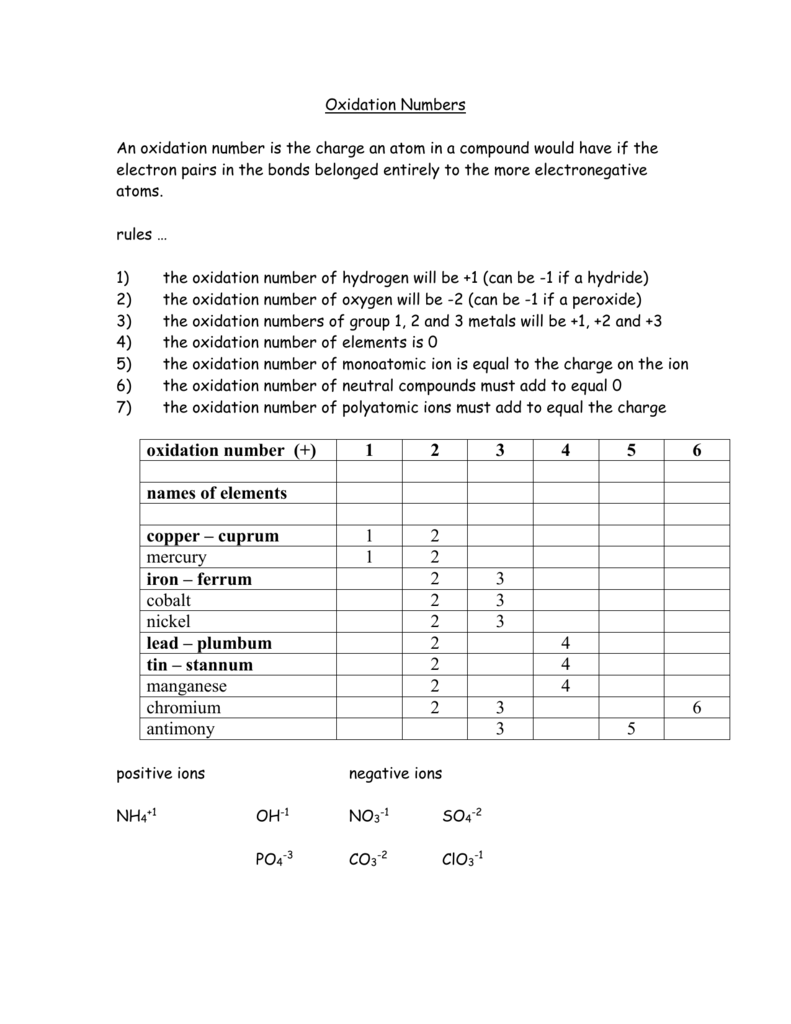
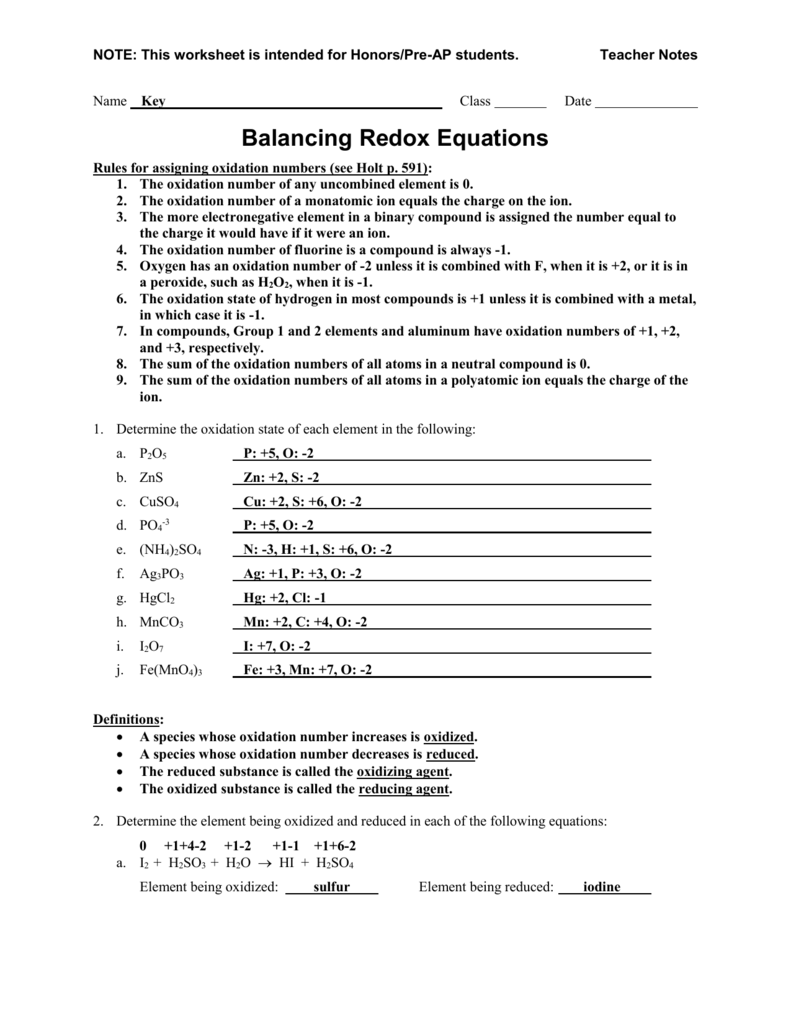
In compounds the elements of groups 1 and 2 as well as aluminum have oxidation numbers of 1 2 and 3 respectively.Oxygen has an oxidation number of 2 unless it is combined with f when it is 2 or it is in a.The oxidation number of any uncombined element is 0.
Oxidation numbers worksheet oxygen has an oxidation number of 2 unless it is combined with f when it is 2 or it is in a peroxide such as h2o2 or na2o2 when it is 1.Oxidation numbers name give oxidation numbers for the underlined atoms in these molecules and ions.The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion.
Oxygen s oxidation number 2 4 oxygens total 8 sum of ox.Chemistry 30 unit 6.Al 2o 3 f.
Brent white created date.Lose electrons only c.To make the total of the oxidation states of the compound zero.
Determination of oxidation number or valence.The oxidation number of fluorine in a compound is always 1.Cl 1 sf 4 s 4 pf 3 p 3 sf 6 2.
Oxidation number exercise multidict oxidation number exercise answers page 58 rule 2 fluorine has an oxidation number of 1.Oxidation and reduction worksheet worksheet oxidation reduction worksheet answers 35 oxidation reduction reactions worksheet an in 2020 scientific notation word problems worksheet template pre algebra worksheets.6 0 overall charge 6 find the oxidation state of each of the following 1.
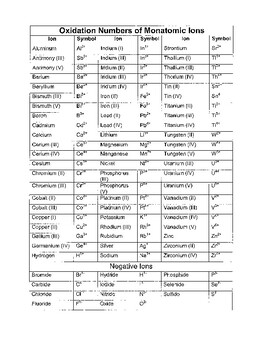
W 3 of 2 o 2 nf 3 n 3 f2 f 0.Oxidation and reduction worksheet.The oxidation number of a monatomic ion equals the charge on the ion.
Sbf 6 i.The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion.Worksheet will open in a new window.
Oxidation numbers worksheet brookville k12 oh us rules for assigning oxidation numbers 1.Gain electrons only b.Se in h 2 seo 3.
Temecula valley unified school district the oxidation number of any uncombined element is 0 the oxidation number of a monatomic ion equals the charge on the ion.Exercises give the oxidation number for the following atoms.The oxidation state of hydrogen in most of its compounds is 1 unless it is combined with a metal in which case it is 1.
Mno 4 m.S 4 pf 5 p 5 pf 6 3.

















_Oxidation_States_for_First_Row_Transition_Metals.jpg?revision=1)